Magnesium Fluoride (MgF2) Pieces Evaporation Materials
Magnesium Fluoride (MgF2) Pieces Overview
We sell these pellets and pieces by unit weight for evaporation use in deposition processes. These approximate materials prices are published to provide budgetary guidelines. Actual prices can vary and may be higher or lower, as determined by availability and market fluctuations. To speak to someone directly about current pricing, please click here .
Magnesium Fluoride (MgF2) General Information
Magnesium fluoride is an inorganic chemical compound with a chemical formula of MgF2. It is white or crystalline in appearance with a melting point of 1,261°C, a density of 3.18 g/cc, and a vapor pressure of 10-4 Torr at 1,000°C. The main application of magnesium fluoride is in optics, due to its distinctive transparency over a wide range of wavelengths. It is evaporated under vacuum to form anti-reflective layers.
Magnesium Fluoride (MgF2) Specifications
| Material Type | Magnesium Fluoride |
| Symbol | MgF2 |
| Color/Appearance | White, Crystalline Solid |
| Melting Point (°C) | 1,261 |
| Theoretical Density (g/cc) | 2.9–3.2 |
| Z Ratio | 0.637 |
| E-Beam | Excellent |
| Thermal Evaporation Techniques |
Boat: Mo, Ta Crucible: Al2O3 |
| E-Beam Crucible Liner Material | FABMATE®, Graphite, Molybdenum |
| Temp. (°C) for Given Vap. Press. (Torr) | 10-4: 1,000 |
| Comments | Substrate temp and rate control important. Reacts with W. Mo OK. |
| Suggested QCM Crystal | Alloy Crystal: 750-1002-G10**** |
**** Suggestion based on previous experience but could vary by process. Contact local KJLC Sales Manager for further information
Empirical Determination of Z-Factor
Unfortunately, Z Factor and Shear Modulus are not readily available for many materials. In this case, the Z-Factor can also be determined empirically using the following method:
- Deposit material until Crystal Life is near 50%, or near the end of life, whichever is sooner.
- Place a new substrate adjacent to the used quartz sensor.
- Set QCM Density to the calibrated value; Tooling to 100%
- Zero thickness
- Deposit approximately 1000 to 5000 A of material on the substrate.
- Use a profilometer or interferometer to measure the actual substrate film thickness.
- Adjust the Z Factor of the instrument until the correct thickness reading is shown.
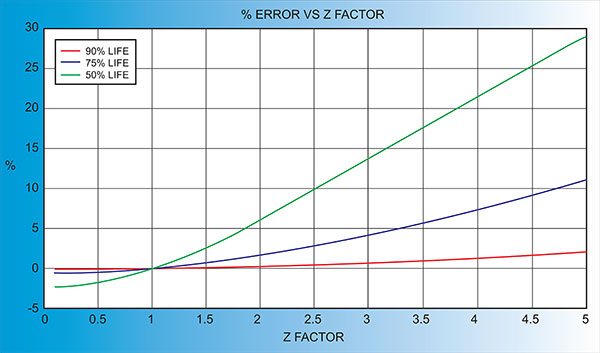
Another alternative is to change crystals frequently and ignore the error. The graph below shows the % Error in Rate/Thickness from using the wrong Z Factor. For a crystal with 90% life, the error is negligible for even large errors in the programmed versus actual Z Factor.
Thermal Evaporation of Magnesium Fluoride (MgF2)
Magnesium fluoride can be thermally evaporated from either a molybdenum or tantalum boat source such as our EVS8B005MO or EVS8B005TA. Tantalum baffle boxes may also be used. It is important to note that the substrate temperature will significantly affect film density and hardness.
With an evaporation temperature of ~950°C and a base pressure for evaporation of 10-6 Torr, we anticipate deposition rates of up to 20 angstroms per second.
E-beam Evaporation of Magnesium Fluoride (MgF2)
Magnesium fluoride is rated excellent for e-beam evaporation. We recommend using a graphite, FABMATE®, or molybdenum crucible liner. It is important to note that the substrate temperature will significantly affect film density and hardness.
We recommend sweeping the beam at low power to uniformly melt the material and avoid hole drilling. Low e-beam power is recommended to avoid material dissociation. With an evaporation temperature of ~950°C and a base pressure for evaporation of 10-6 Torr, we anticipate deposition rates of up to 20 angstroms per second. We expect good adhesion to most substrates. Yttrium oxide (Y2O3) can be used as thin adhesion layer when necessary.
Another key process note is to consider the fill volume in the e-beam application because we find that the melt level of a material in a crucible directly affects the success of the crucible liner. Overfilling the crucible will cause the material to spill over and create an electrical short between the liner and the hearth. The outcome is cracking in the crucible. This is the most common cause of crucible liner failure. Placing too little material in the crucible or allowing the melt level to get too low can be detrimental to the process as well. When the melt level is below 30%, the e-beam is likely to strike the bottom or walls of the crucible which immediately results in breakage. Our recommendation is to fill the crucible between 2/3 and 3/4 full to prevent these difficulties.
Crucible liners should be stored in a cool, dry place and always handled with gloves or forceps.
See highlighted results that match your result in the table below.
Ordering Table
| More Info | Material | Description | Size | Quantity | Purity | Part Number | Price | In Stock | Add To Cart |
|---|---|---|---|---|---|---|---|---|---|
| More Info | Material | Description | Size | Quantity | Purity | Part Number | Price | In Stock | Add To Cart |
| Magnesium Fluoride |
MAGNESIUM FLUORIDE PIECES, |
1mm - 2.5mm Pieces | 1 kg | 99.99% | EVMMGF-1-2.5 | $222.00 |
|
||
| Magnesium Fluoride |
MAGNESIUM FLUORIDE PIECES, |
1mm - 2.5mm Pieces | 25 g | 99.99% | EVMMGF-1-2.5A | P.O.R. |
|
||
| Magnesium Fluoride |
MAGNESIUM FLUORIDE PIECES, |
1mm - 2.5mm Pieces | 50 g | 99.99% | EVMMGF-1-2.5B | P.O.R. |
|
||
| Magnesium Fluoride |
MAGNESIUM FLUORIDE PIECES, |
1mm - 2.5mm Pieces | 500 g | 99.99% | EVMMGF-1-2.5T | P.O.R. |
|
||
| Magnesium Fluoride |
MAGNESIUM FLUORIDE PIECES, |
1mm - 4mm Pieces | 500 g | 99.995-99.999% | EVMMGF501-4T | P.O.R. |
|
||
| Magnesium Fluoride |
MAGNESIUM FLUORIDE PIECES, |
3mm - 6mm Pieces | 50 g | 99.9% | EVMMGF-1113B | $62.00 |
|
||
| Magnesium Fluoride |
MAGNESIUM FLUORIDE PIECES, |
3mm - 6mm Pieces | 100 g | 99.9% | EVMMGF-1113D | $83.00 |
|
||
| Magnesium Fluoride |
MAGNESIUM FLUORIDE PIECES, |
3mm - 6mm Pieces | 500 g | 99.9% | EVMMGF-1113T | $290.00 |
|
||
| Magnesium Fluoride |
MAGNESIUM FLUORIDE PIECES, |
3mm - 6mm Pieces | 1 kg | 99.9% | EVMMGF1113KG | P.O.R. |
|