Yttrium Fluoride YF3 Evaporation Process Notes
Yttrium fluoride is an inorganic chemical compound with a chemical formula of YF3. It is white in appearance with a melting point of 1,387°C and a density of 4.01 g/cc. It is commonly found in various glasses and ceramics. Some users will utilize yttrium fluoride in place of thorium fluoride (ThF4) which is radioactive. Yttrium fluoride is evaporated under vacuum to form antireflective layers in optics and dielectric layers for passive components fabrication.
Yttrium Fluoride YF3 Specifications
| Material Type | Yttrium Fluoride |
| Symbol | YF3 |
| Color/Appearance | White, Solid |
| Melting Point (°C) | 1,387 |
| Theoretical Density (g/cc) | 4.01 |
| Sputter | RF |
| E-Beam Crucible Liner Material | Tantalum, Molybdenum |
Z-Factors
Empirical Determination of Z-Factor
Unfortunately, Z Factor and Shear Modulus are not readily available for many materials. In this case, the Z-Factor can also be determined empirically using the following method:
- Deposit material until Crystal Life is near 50%, or near the end of life, whichever is sooner.
- Place a new substrate adjacent to the used quartz sensor.
- Set QCM Density to the calibrated value; Tooling to 100%
- Zero thickness
- Deposit approximately 1000 to 5000 A of material on the substrate.
- Use a profilometer or interferometer to measure the actual substrate film thickness.
- Adjust the Z Factor of the instrument until the correct thickness reading is shown.
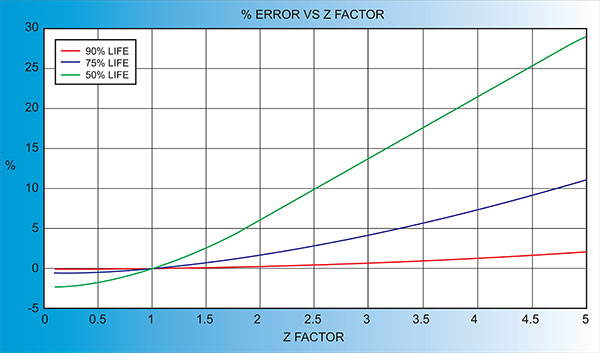
Another alternative is to change crystals frequently and ignore the error. The graph below shows the % Error in Rate/Thickness from using the wrong Z Factor. For a crystal with 90% life, the error is negligible for even large errors in the programmed versus actual Z Factor.