Lithium Fluoride LiF Evaporation Process Notes
Lithium fluoride is an inorganic compound with a formula of LiF. It has a density of 2.64 g/cc, a melting point of 845°C, and a vapor pressure of 10-4 Torr at 1,180°C. It is crystalline in appearance and white in color. One of its main uses is as a component of molten salts and it is evaporated under vacuum to form anti-reflective layers for optical coatings.
Lithium Fluoride LiF Specifications
| Material Type | Lithium Fluoride |
| Symbol | LiF |
| Color/Appearance | White, Crystalline Solid |
| Melting Point (°C) | 845 |
| Theoretical Density (g/cc) | 2.64 |
| Sputter | RF |
| Z Ratio | 0.778 |
| E-Beam | Good |
| Thermal Evaporation Techniques |
Boat: Ni, Ta, Mo, W Crucible: Al2O3 |
| E-Beam Crucible Liner Material | Tantalum, Tungsten, Molybdenum |
| Temp. (°C) for Given Vap. Press. (Torr) |
10-8: 875 10-6: 1,020 10-4: 1,180 |
| UN Number | 3288 |
| Comments | Rate control important for optical films. Preheat gently to outgas. |
Z-Factors
Empirical Determination of Z-Factor
Unfortunately, Z Factor and Shear Modulus are not readily available for many materials. In this case, the Z-Factor can also be determined empirically using the following method:
- Deposit material until Crystal Life is near 50%, or near the end of life, whichever is sooner.
- Place a new substrate adjacent to the used quartz sensor.
- Set QCM Density to the calibrated value; Tooling to 100%
- Zero thickness
- Deposit approximately 1000 to 5000 A of material on the substrate.
- Use a profilometer or interferometer to measure the actual substrate film thickness.
- Adjust the Z Factor of the instrument until the correct thickness reading is shown.
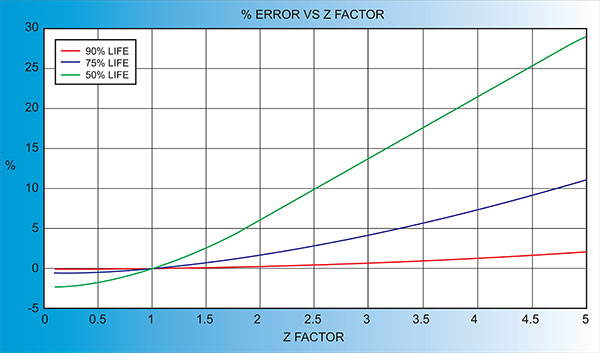
Another alternative is to change crystals frequently and ignore the error. The graph below shows the % Error in Rate/Thickness from using the wrong Z Factor. For a crystal with 90% life, the error is negligible for even large errors in the programmed versus actual Z Factor.